Women's health startup Daye expands its revolutionary diagnostic tampon to include STI screening
Gynaecological health startup Daye launches first of its kind at-home tampon-based STI screening kit
Pioneering kit enables women and AFAB individuals to test for STIs, such as chlamydia and gonorrhoea, from the comfort of their own homes using a tampon instead of an uncomfortable swab
Service will include personalised aftercare, including treatments and consultations with sexual health nurses, gynaecologists and fertility specialists
Award-winning gynaecological health startup Daye is expanding its revolutionary tampon-based at-home vaginal microbiome screening kit, which was launched one year ago, to include STI screening.
The first-of-its-kind tampon-based at-home STI screening kit will help speed up diagnosis and treatment, particularly among patient groups who are anxious or embarrassed about getting a test.
The pioneering new “STI Diagnostic Tampon” utilises cutting-edge PCR testing technology to allow women and assigned female at birth (AFAB) individuals to test for chlamydia, gonorrhoea, trichomonas, mycoplasma and ureaplasma from the comfort and convenience of their own home.
The launch follows clinical trials with 600 patients. The samples will be analysed in a UKAS-accredited lab, following CQC-approved screening methodologies using a PCR-based, CE-marked diagnostic assay. The Daye Diagnostic Kit is registered with the MHRA.
As part of the service, Daye will also provide personalised aftercare, including prescription treatments such as antibiotics and antivirals, and consultations with sexual health nurses, gynaecologists and fertility specialists.
There has been a 24% increase in sexually transmitted infections compared to the previous year, according to the UK Health Security Agency[1], and women are statistically more at risk than men due to the vaginal physiology.
Despite this, fewer people are getting tested for STIs, with over half of Brits admitting they’ve never been for a sexual health check[2].
STIs still carry a lot of stigma, yet are often perfectly treatable if caught in time. However, testing is still thought to be awkward, embarrassing and uncomfortable[3].
Low rates of testing mean that many women and AFAB individuals may be unaware that they have an STI as 70% of them are asymptomatic.
Untreated STIs pose risks to long-term health and fertility. Chlamydia and gonorrhoea are two of the leading preventable causes of infertility and ectopic pregnancies. Meanwhile, mother-to-child transmission of STIs can result in stillbirth, neonatal death, low birth weight and other health complications.
Untreated STIs can also lead to chronic pelvic pain and pelvic inflammatory disease.
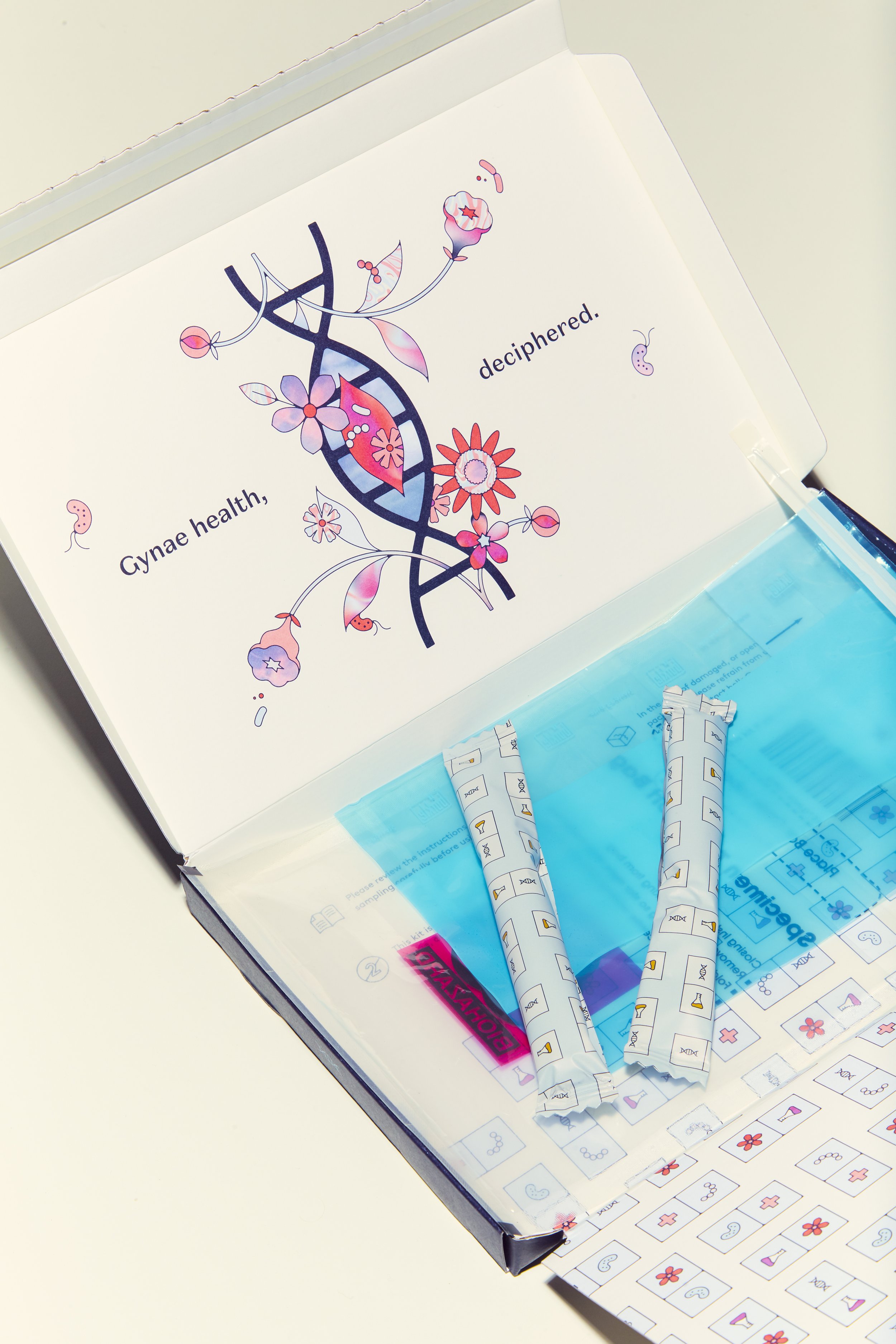



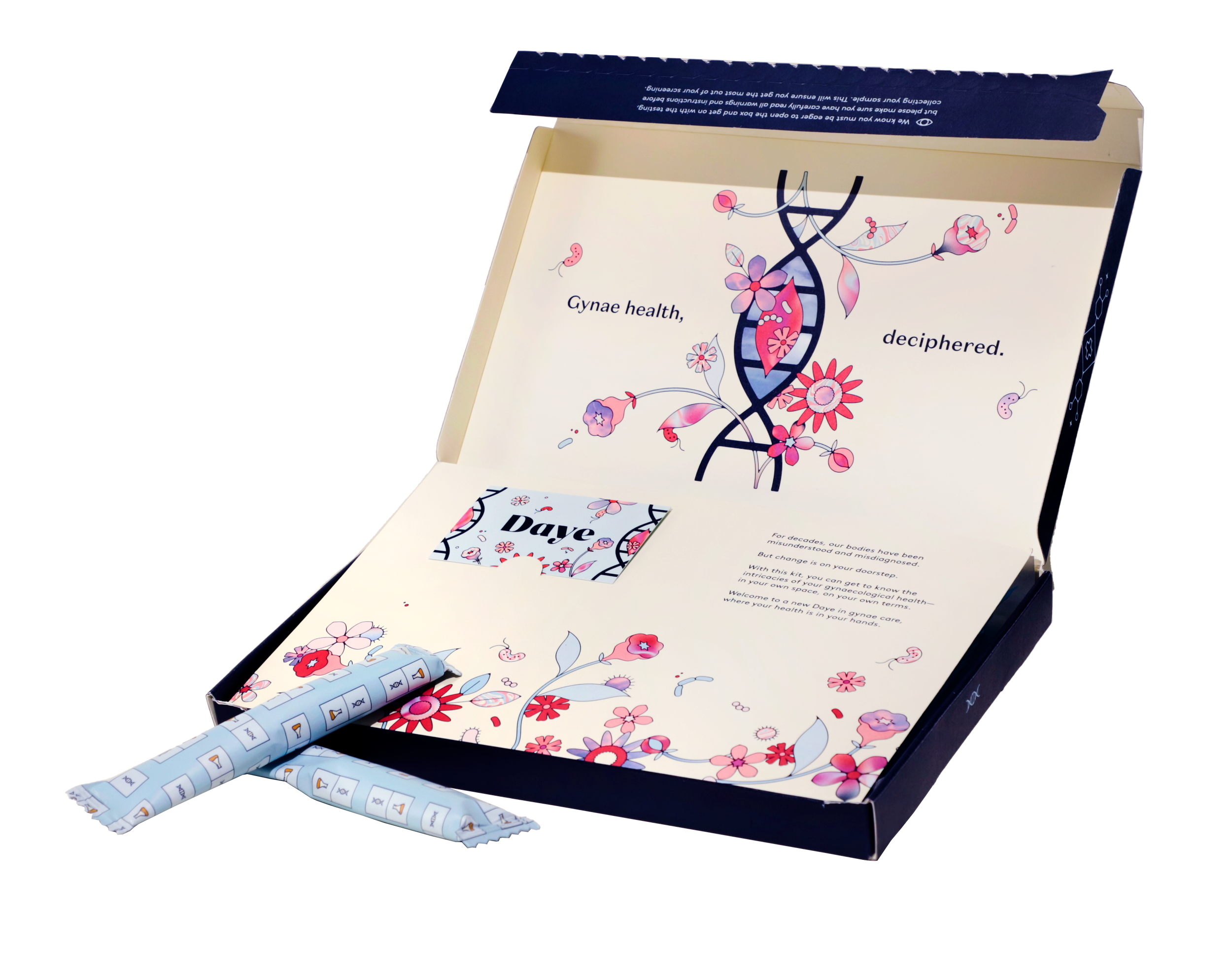
Daye’s new STI Diagnostic Tampon will allow people to collect a sample in the comfort of their own home and then discreetly send it to a lab that will test for multiple STIs, returning results within five working days.
It uses PCR (Polymerase Chain Reaction) technologies, which have revolutionised the screening and diagnosis of STIs by detecting even trace amounts of genetic material from pathogens. The specificity of PCRs ensures that false-positive results are reduced, providing a more reliable diagnosis, and it can simultaneously detect multiple pathogens in a single sample.
Used just like a normal tampon, Daye’s unique STI Diagnostic Tampon collects more vaginal fluid than a standard swab and covers a larger surface area, making it more accurate than a swab and more comfortable than a speculum. This means it gets the most comprehensive results possible for common STIs – plus the ones you might not know about.
Daye’s research has found that patients and clinicians alike prefer the use of a tampon over a swab[4].
The tampon has a smooth sugarcane applicator for pain-free and comfortable insertion. The applicator allows the user to reach their cervix by themselves without a speculum.
The STI Diagnostic Tampon is MHRA registered, and Daye is certified under ISO13485 and GMP for the manufacture, clinical validation and post-market surveillance of the Diagnostic Tampon.
Valentina Milanova, Founder of Daye, comments: “Despite living in a world where over a million people get an STI every day, STI testing has chronically low rates post-COVID-19, and as a result, many women and AFAB individuals could have an infection unknowingly because they have no symptoms.
“Our STI Diagnostic Tampon makes STI testing extremely easy, comfortable and discrete. We hope our approach will end the “STIgma", revolutionise STI testing and lead to a dramatic uptick in the number of women getting checked, helping them protect their long-term health and fertility.”
About Daye
Daye is a female-founded gynaecological health startup that is fast becoming the leading destination for menstrual pain management, vaginal health, and hormonal awareness.
Founded in 2017, the company is on a mission to overcome historical gender biases and close the gender gap in medical research and innovation. Daye aims to empower women and assigned female at birth (AFAB) individuals to take control of their gynaecological health by pioneering gynocentric research and creating revolutionary products and services for period pain, menstrual care and at-home testing for STIs, HPV and vaginal infections.
Daye was recognised by WIRED as one of London’s hottest startups in 2022 and 2019, and Daye’s founder, Valentina Milanova, was named on the 2023 Forbes 30Under30 Europe list. For more information, please visit yourdaye.com and @yourdaye on Instagram, Facebook, TikTok and Twitter.

